Pioneering
minimallyinvasive
solutions for ENTs


Pioneering
minimallyinvasive
solutions for ENTs

At Spirair®, we recognize the unmet need for minimally invasive solutions that help ENTs advance care for their patients by providing strong outcomes and a better patient experience.
That’s why we are committed to pioneering new solutions that can easily integrate in any ENT’s treatment algorithms so they can help more of their patients breathe better.
Minimally invasive.
Massively impactful.

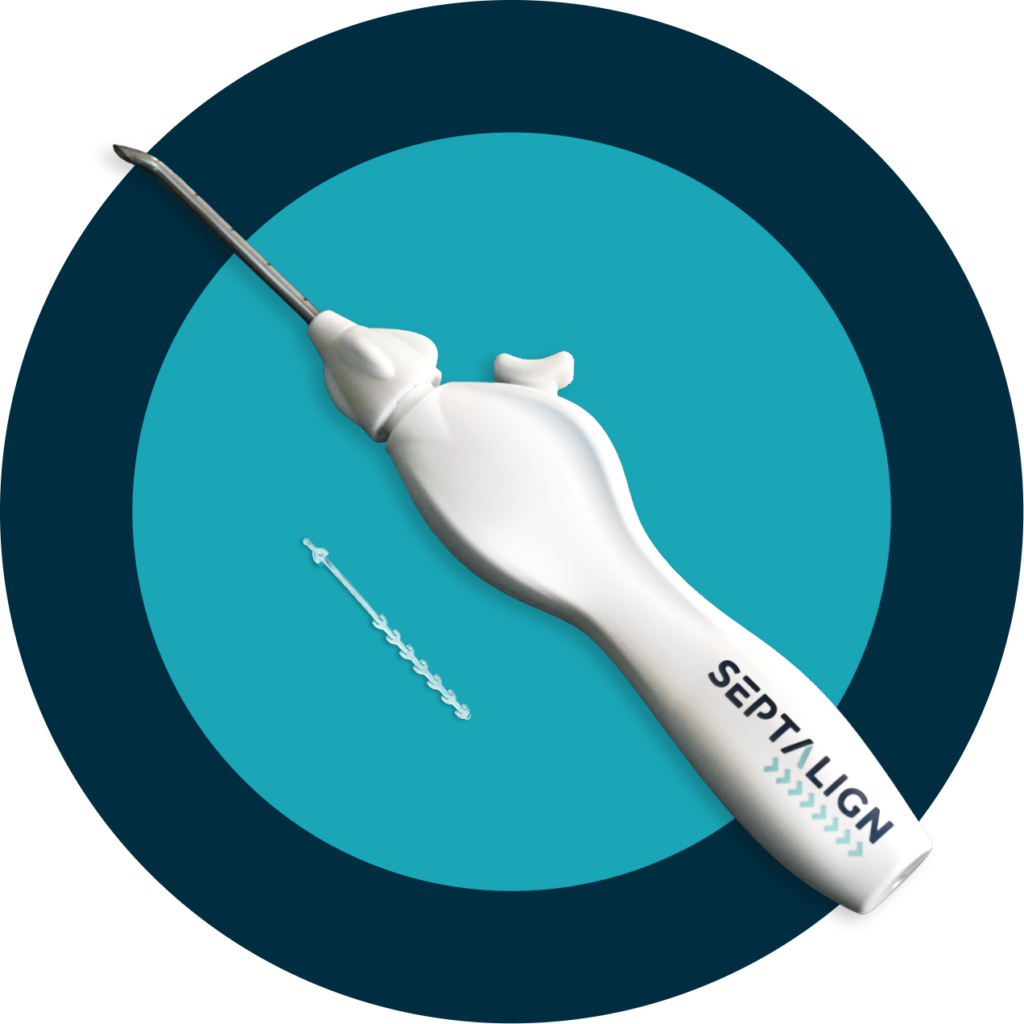
Minimally invasive.
Massively impactful.



“After the SeptAlign procedure, I went back to work the next day. I liked that there wasn’t a long recovery, and now I can breathe so much better.”*
-SeptAlign Patient
*Individual results may vary
“After the SeptAlign procedure, I went back to work the next day. I liked that there wasn’t a long recovery, and now I can breathe so much better.”*
-SeptAlign Patient
*Individual results may vary

Medialization
Made Simple.


Medialization
Made Simple.


Ready to learn more about TurbAlign?
